The FDA’s Division of Drug Marketing, Advertising and Communications (DDMAC) recently issued warning letters to fourteen leading drug companies for misleading and misbranded advertising practices on internet search engines for prescription medications. To date, this ruling does not impact over-the-counter medications or supplements on internet search engines. This POV outlines guidelines to take in order to respond to the FDA’s actions regarding paid search engine advertising and organic search implications.
Paid Search Implications
The FDA warnings were the result of claims being made in paid search ads without corresponding risk information being provided in the ads.
Since paid search ads provide very limited space for communicating an advertising message (25 characters in the ad title, and 70 characters in the body of the ad), it is unfeasible to include all the necessary risk information in the advertisement.
The anatomy of a paid search ad:
Ad Title (25 characters)
Description Line 1: (35 characters)
Description Line 2: (35 characters)
Display URL: (35 characters)
Therefore, paid search ads and keyphrases for pharmaceutical products must now follow very strict guidelines as outlined below:
Guidelines for Branded Paid Search Advertising:
Branded advertising guidelines apply to any paid search ad that meets one or more of the following criteria:
- Ad contains the brand name of the product (ex. Viagra, Crestor, Advair, etc.). It doesn’t matter where the name appears in the ad, even if it’s only in the display URL.
- Ad contains the generic name of the product anywhere in the ad.
- The ad contains language that makes it possible to identify the brand name of a drug. (Ex. “The only infusion treatment for multiple sclerosis”).
Guideline 1: Ad Copy
Branded ads should follow the FDA’s guidelines for “reminder ads“. They can call attention to the name of the drug product but do not include indications or dosage recommendations for use of the drug product. Reminder advertisements cannot make a representation about the product or suggest a use for the product.
In short, branded ads cannot contain the indication(s) of the drug.
| Example of a Branded Ad in Compliance | Example of a Branded Ad Not in Compliance
|
| Viagra® Official Site
Get the facts on Viagra (sildenafil citrate). |
Viagra® Official Site
An effective treatment for Erectile Dysfunction. |
Guideline 2: Keyphrases
The search terms associated with branded ads must be limited to branded terms. No search terms that make a claim should be linked to a branded advertisement. To control this, the match type for keyphrases associated with branded ads must be set to “exact” match only.
- Do not use the following match types on Google: “broad”, “phrase”
- Do not use the following match types on Yahoo: “advanced”
- Do not use the following match types on MSN/Live: “broad”, “phrase”
Guideline 3: Landing Pages
The term “landing page” refers to the website page a searcher is taken to when they click on a paid search ad. A branded ad can lead to either a branded or unbranded website. For example, the ad below could link searchers to a page on Zoloft.com.
Zoloft
Learn About Zoloft. Find
Questions to Ask Your Doctor.
Guidelines for Unbranded Paid Search Advertising:
An unbranded paid search ad is defined as follows:
- The ad does not contain the brand name of the drug either in the title, body, or display URL of the ad.
- The ad does not contain the generic name of the drug anywhere in the ad.
- The ad does not contain language that makes it possible to identify the brand name of a drug. (Ex. “The only infusion treatment for multiple sclerosis”).
Guideline 1: Ad Copy
Unbranded ads may include mention of a condition, disease, or treatment, as long as they abide by the definition of an unbranded ad as outlined above.
| Example of an Unbranded Ad in Compliance | Example of an Unbranded Ad Not in Compliance
|
| Hypertension Treatment
Learn About Treatment Options For Hypertension. |
Hypertension Treatment
Learn About Treatment Options For Hypertension. |
Guideline 2: Keyphrases
The search terms associated with unbranded ads should be limited to unbranded terms. To control this, the match type for keyphrases associated with unbranded ads must be set to “exact” match only.
- Do not use the following match types on Google: “broad”, “phrase”
- Do not use the following match types on Yahoo: “advanced”
- Do not use the following match types on MSN/Live: “broad”, “phrase”
Guideline 3: Landing Pages
An unbranded ad can link to either a branded (using a vanity URL with safety information on the landing page) or unbranded website. For example, the ad below could link searchers to a page on Zoloft.com, or to a page on an unbranded site such as www.depression.com.
Depression Medication
Learn About An Effective
Treatment For Depression.
Catalyst recommends linking unbranded ads to unbranded websites since searchers who search on unbranded terms are most likely looking for generic or unbiased information about treatments for a particular condition and the vanity URL will lead the consumer to believe it is an unbraded site. Leading searchers to a branded site, from an unbranded ad, will likely result in high bounce rates and thus lead to lower ROIs.
Business impact: This practice will reduce awareness, traffic to the site and engagement with consumers.
Consumer impact: Consumers will be less aware of pharmaceutical products to meet their health needs at a point of receptivity.
Next Steps:
As a result of the FDA’s recent emphasis on the enforcement of advertising regulations, Catalyst online is working with each of the major search engines (Google, Yahoo, MSN) to determine if new methods can be developed for displaying paid search advertising to enable risk information to be displayed. Some of the ideas that we are pursuing, include:
- The addition of an interstitial page that displays risk information immediately upon a searcher clicking on a paid ad (however, has an organic search impact).
- A pop-up display containing risk information when a searcher moves their mouse over a paid search ad.
- The addition of link within the paid search ad that will take the user to a page containing the risk information for the product.
- And more.
Organic Search Implications
Guideline: No change at this time for organic listings
In addition to the impact on paid search, which is a paid media buy, there is accompanying speculation on whether the recent FDA warning letters will expand to impact organic search listings. This is particularly relevant for brands conducting simultaneous paid and organic campaigns, as well as brands seeking to offset any loss in paid search traffic with their organic listings.
The current status is that the FDA HAS NOT sent letters to any company based on their organic listings. And there’s good reason. First, your organic listing is not a paid media buy. Second, your organic listing is controlled, in large part, by the search engine’s (e.g. Google’s) algorithm, which decides who will rank for a particular search term and what is presented to a searcher. Therefore, we would recommend brands refrain from changing their SEO campaign or search rankings until the FDA clarifies its position.
Recently we have received requests by clients to develop a scenario, including pros and cons, in the event internal regulation were to consider applying the new paid rules to organic listings. The following is our opinion on what that would look like and the business ramifications.
Scenarios for implementing paid rules on organic listings:
There are only three elements from your web page that appears on a results page of a search; Title, Description and URL (the domain and file name of the asset).
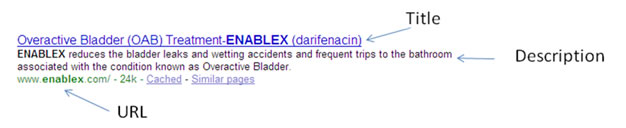
Consideration of Title Tag:
HTML Title: Typically appearing at the top of the browser window, this text is commonly used in search engine listings as the link-text pointing to the page in question. It also displays in the Search engines result page after a search.
To match the paid rules, the generic drug name and brand name would need to appear together in the title, but the indication can not appear in the title. You can control this by adjusting your title tag on the webpage.
Business impact: This has a negative impact on search engine results; sites will no longer rank for the indication.
Consumer Impact: The Search Engine Results Page (SERP) will be filled with non-pharma results, including herbal remedies and online drug stores.
Consideration of Meta Description in combination with title:
Meta Description: A small piece of text that describes the page in question and often accounts for the ’snippet’ or descriptive text in a search listing. Although you can try to influence it, the engine’s algorithm ultimately determines which text it will use for the description.
Again, to mirror the paid rules, if the name of the drug is used in the title or description of the URL, there can be no mention of the indications. The example below shows two ’snippets’. In the first you see that the brand Enablex is clearly coupled with the description highlighting its treatment for overactive bladder. Google choose this information from the homepage. In the second, you see that Google has decided to piece together separate sentences to create a description for Asacol.
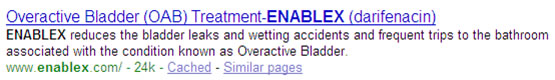
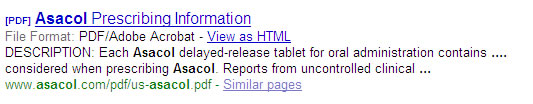
Business impact: In this case, changing the description, even if possible, would dramatically affect the click-through to the site. The description is usually the determining factor on whether you decide to click to a site or skip to the next listing.
Consumer impact: Confusion and devaluation of the SERP results.
Consideration of the URL (Branded vs. Unbranded):
In the paid ruling, the FDA stated that the URL, if branded, acts as brand impression and therefore triggers the need for either fair balance or the omission of the condition in the remaining copy. In the following example the presence of Asacol in the URL www.asacol.com/glossary.jsp , would cause this listing to be questioned.
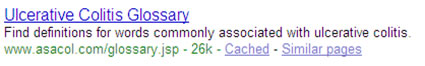
Business impact: The only way to avoid this paid rule is to use an unbranded URL (e.g. www.UC-Glossary.com). If you choose this path, your brand site would never rank for your disease state. In addition, other OTC, mock-treatment or home remedies would replace your organic listing and harvest all of the traffic you were formerly receiving. This is a critical issue. Also, you would potentially need to build a separate non-branded site for each branded site, which is more costly to maintain.
Consumer impact: Consumers will be less aware of pharmaceutical products to meet their health needs at a point of receptivity.
Next Steps:
- Until the FDA determines that the organic listing, which is controlled by the search engines, is an area of concern - our opinion is to maintain your organic listing as normal.
- If you are going to apply the paid rules to the organic listing, please contact Catalyst online to ensure you are not inadvertently creating major site traffic or search issues for your site.
If you (or your regulatory team) have any questions regarding this POV, please contact Tim Breen at Catalyst online at tbreen@CatalystSearchMarketing.com, or at 617.244.6679.


2 Comments
Hi, nice posts there thank’s for the interesting information
thank’s for the interesting information
What a fantastic article. Got some new information that can prove very important, cheers for sharing …